by Federica Bellerba, Andrea Caldarone, Sara Gandini
Department of Experimental Oncology (DEO), European Institute of Oncology (IEO), IRCCS, Milano
Table of Contents
References
Introduction
Understanding the evolution dynamics of the COVID-19 pandemic in the coming months is a matter of great importance and urgency, as governments around the world will have to make fundamental decisions based on what is known about the mechanisms of transmission of the virus and its survival in the environment.
Experts have been studying the role of weather and environmental factors on the evolution of the pandemic from the outset, suggesting that Sars-CoV-2 may have the same seasonal behaviour observed in many infectious diseases and in other strains of coronavirus.
The virus first emerged in China in December 2019 and rapidly spread in the northern hemisphere, moving from east to west, in regions characterized by a cool and temperate climate, typical of the winter season. Currently, as these regions are heading towards the summer season and a reduction in infections, in the southern hemisphere the winter season is approaching and a considerable increase in cases is being observed. This could suggest a climatic susceptibility of the virus and raise concerns for the upcoming cold seasons in which, if this were confirmed, a new rise of infections could occur.
Epidemiology of COVID-19
On December 31, 2019, the World Health Organization (WHO) declared an outbreak of pneumonia cases with unknown cause in the city of Wuhan, in the Chinese province of Hubei. On January 9, 2020 the WHO announced that the Chinese authorities had identified a new strain of coronavirus that had never been found in humans, which could be the cause of these pneumonia cases. The virus was later classified under the name Sars-CoV-2 and the respiratory disease caused by it was called COVID-19.
From then on, the virus has spread all over the world, affecting more than 180 countries. On 11 March 2020, WHO declared the novel SARS-CoV-2 coronavirus infection a pandemic. Currently, at the end of July 2020, the total number of diagnosed cases in the world is more than 16 million and more than 660 000 deaths were reported. The countries with the highest number of confirmed cases are the United States (over 4 million), Brazil (over 2.4 million), India (over 1.5 million), Russia (over 800 thousand), South Africa (over 450 thousand), Mexico (over 400 thousand), Peru (over 350 thousand), Chile (over 350 thousand), the United Kingdom (over 300 thousand) and Iran (over 250 thousand). The countries with the highest number of recorded deaths are the United States (over 148 thousand), Brazil (over 88 thousand), the United Kingdom (over 45 thousand), Mexico (over 44 thousand), Italy (over 35 thousand), India (over 34 thousand), France (over 30 thousand), Spain (over 28 thousand), Peru (over 18 thousand) and Iran (over 15 thousand9 (Figure 1).
America is the continent that is currently facing the higher number of cases, followed by Asia and Africa, while Europe, which was heavily affected during the first phase of the pandemic in spring, is now having a gradual reduction in infections1.
Figure 1 Case and deaths by continent
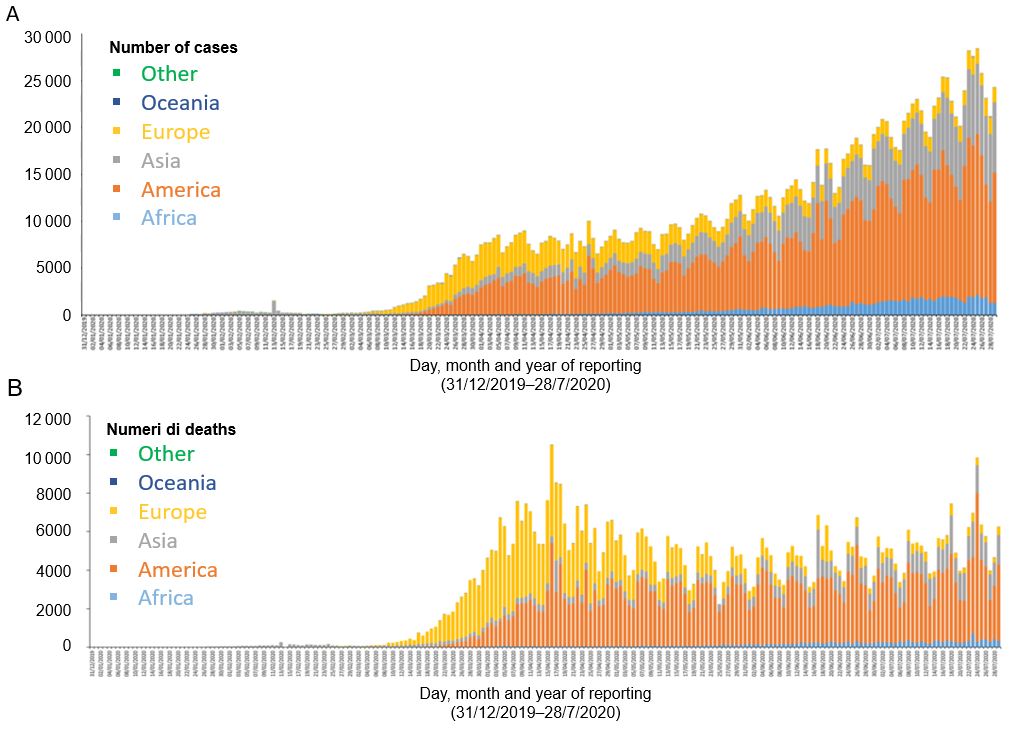
A Distribution of COVID-19 cases by continent (according to the applied case definition and testing strategies in the affected countries), as of 30 July 2020. B Distribution of COVID-19 deaths by continent, as of 30 July 2020. [Source: European Centre for Disease Prevention and Control]
Symptoms of COVID-19
COVID-19 symptoms can widely vary, ranging from mild to severe illness. The most common symptoms are fever, dry cough and tiredness; less common symptoms include nasal congestion, headache, conjunctivitis, sore throat, diarrhea, loss of taste or smell and a rash or discoloration of the fingers or toes2. Symptoms typically occur 2–14 days after exposure to the virus, for this reason suspected cases are required to observe a 14-day quarantine period. Symptoms may initially appear in a mild form and then intensify in the following −7 days, leading to breathing difficulties or pneumonia3. About 80% of confirmed cases recover from the disease without undergoing hospital treatment, while 1 in 5 people with COVID-19 gets seriously ill and develops breathing problems2.
COVID-19 mortality
In order to understand the severity of this pandemic, it is fundamental to know the risk of death of an individual affected by COVID-19, i.e. the probability that a person affected by COVID-19 will die. Over the past few months, three different measures have often been reported:
- The case fatality rate (CFR);
- The mortality rate;
- The infection fatality rate (IFR).
These ratios, although conceptually very different, are frequently mistaken as synonyms:
- The CFR is the ratio between the number of confirmed deaths and the total number of confirmed cases. It cannot be considered as the probability of an affected person to die, because it includes only confirmed cases, leaving out the pool of untested subjects with COVID-19 (usually asymptomatic and pauci-symptomatic individuals, who are difficult to track);
- The mortality rate is the ratio between the number of confirmed deaths and the number of subjects in the population; it measures the probability that any individual from the population will die of COVID-19. It does not return the probability of an affected person to die, but provides an estimate of the risk of death at the population level;
- The IFR is the ratio between the total number of confirmed deaths and the total number of subjects who were exposed to the virus; therefore, it measures the probability that an individual affected by COVID-19 will die. In order to calculate this quantity, it is necessary to know both the real number of cases and the real number of deaths.
Based on these definitions, the CFR, which considers the total number of confirmed cases, is also called apparent lethality, whereas the IFR, which considers the total number of cases, including the untested asymptomatic and pauci–symptomatic individuals, is also referred as real lethality.
Currently, the CFR is the most widely used measure. It is often mistakenly reported as a single, constant quantity, but it strongly depends on several factors, such as the reporting period, the characteristics of the affected population and, most of all, the screening policy.
As the CFR strongly depends on the evolution of the pandemic, it can be both an overestimation and an underestimation of the IFR.
A country that is facing an exponential growth of infections will likely have both the health and the surveillance systems under stress, and will probably be able to test only hospitalized or highly symptomatic subjects. The diagnosed cases will therefore be those most at risk of dying and this will lead to a higher CFR. In these circumstances, the CFR will be an overestimate of the IFR, as it does not include the pool of asymptomatic and pauci-symptomatic cases.
This implies that the CFR will be close to the real value of the IFR only when screening campaigns include the asymptomatic population, but this strategy is very complex to implement during acute phases of the virus transmission.
On the other hand, the CFR also depends on the number of confirmed deaths. As the pandemic is still ongoing, some people who are currently affected by COVID-19 will die of this disease in the future. This means that the current CFR is based on an underestimation of the deaths that will be recorded among the current confirmed cases, as some cases of today have not yet died but will do so in the future. Furthermore, it is also unlikely that all deaths from COVID-19 have been ascertained through diagnostic testing, so this is an additional source of bias that leads the CFR to underestimate the IFR. An example is what happened during the Sars-CoV epidemic of 2003: in the early stages of the epidemic, the CFR was reported to be about 3–5%, but rose to be about 10% at the end of the epidemic4,5.
This means that it will be possible to have a realistic estimate of the actual CFR only at the end of the pandemic. In the meantime, caution must be exercised in the interpretation of current CFRs.
Nowadays, CFRs vary widely from country to country and range from 0.1% in Singapore to 27.9% in Yemen6. A meta-analysis conducted by the Center for Evidence-Based Medicine of the University of Oxford, updated on 26 May 2020, reported an heterogeneity equal to 100% between countries’ CFRs7.
The age of the tested population could play an important role in explaining this great difference. It is known that the CFR value increases with increasing age, for this reason the age distribution of a population could significantly affect its overall CFR. An example is provided by Italy, which has one of the highest apparent lethality but, at the same time, has the oldest population in the world after Japan. Age could therefore be one of the key factors to explain this anomaly. For 9 countries that have published COVID-19 CFRs by age group (Italy, Holland, Spain, France, China, Switzerland, South Korea, the United States and Germany), the overall CFR was transformed into a standardized CFR taking into account the age distribution of the population (Age-expected CFR) and into a standardized CFR based on a standard age distribution, comparable between countries (Age-standardized CFR). These estimates were calculated under the assumption that all age groups have the same probability to be infected. Taking into account the age distribution of the population greatly reduced the differences between countries: for example, the CFRs of Italy and Spain halved, respectively, from 9.2 to 4.6 and from 6.0 to 2.9, the CFR of the Netherlands decreased by almost two thirds, from 7.4 to 2.6, whereas the CFR of Germany rose from 0.7 to 1.5 (Figura 2). These results suggest that the lethality estimates are biased by the age of the tested individuals and, consequently, strongly depend on the screening strategies adopted by each country8.
Figure 2 Case-fatality rates and age-expected case-fatality rates
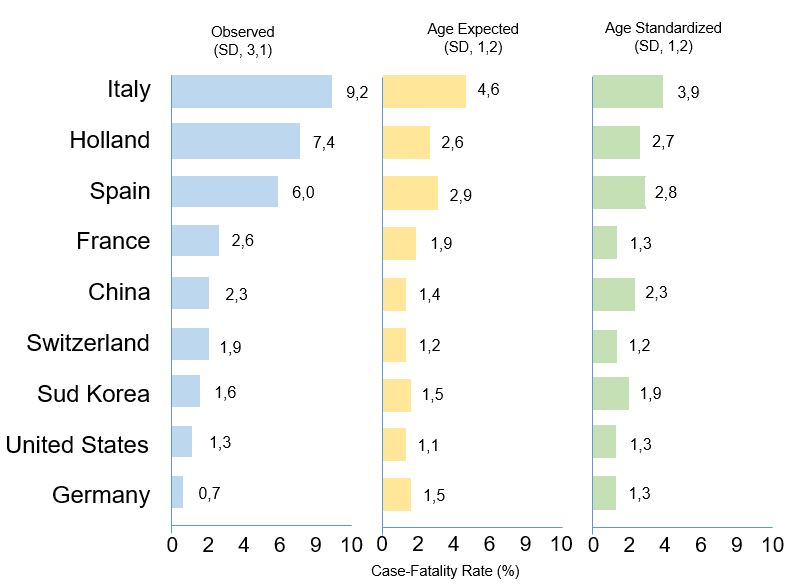
The first plot shows observed COVID-19 case-fatality rates; the second plot shows age-expected COVID-19 case-fatality rates, i.e. case-fatality rates that use the age distribution of the country’s general population; the third plot shows age-standardized COVID-19 case-fatality rates, i.e. case-fatality rates that use the mean age distribution of diagnosed cases across the 9 countries as the standard. [Source: Sudharsanan N, Didzun O, Bärnighausen T, Geldsetzer P. The Contribution of the Age Distribution of Cases to COVID-19 Case Fatality Across Countries: A 9-Country Demographic Study. Ann Intern Med. 2020 Jul 22;M20-2973.]
Consequently, relying on CFR estimates may not provide an accurate measure of the severity of the disease and of the risk of dying of COVID-19. Despite this, it is a very important indicator as its temporal variability and geographical dependency could help understand the moment of the pandemic in which each country is at and provide information on its testing policy6.
Unlike CFR and IFR, the mortality rate is a solid epidemiological indicator as it provides a measure at the population level and allows for comparisons over time and at a territorial level. Through these comparisons, it provides very useful information both on the general state of health of the population and on the spread mechanisms of diseases, such as COVID-199.
In Italy, during the pandemic, there were about 40 thousand more deaths than those expected in previous years10. Despite this, similar excess mortality was also observed in the past: in 2015, for example, 50 thousand more deaths were recorded than in the previous year, due to the flu peak of the winter season and the heat waves in the summer season11.
In order to have a clearer understanding of how COVID-19 has affected the population in terms of mortality, it is therefore necessary to investigate what has happened at a territorial level.
Comparing mortality data between January and May 2020 at the regional level with those of the same period in 2015, it is clear how the pandemic dramatically hit the northern regions—especially Lombardy—while it had a much more contained effect in the centre and in the south. This means that in the most affected provinces, such as Brescia, Cremona and Bergamo, there will likely be a strong reduction in life expectancy in the coming years, whereas minor consequences are expected in the rest of the national territory (Figure 3).
Figure 3 Distribution of daily deaths from 1 January to 31 May in 2015 and in 2020 in four Italian regions: Lombardy, Lazio, Campania, Sicily.
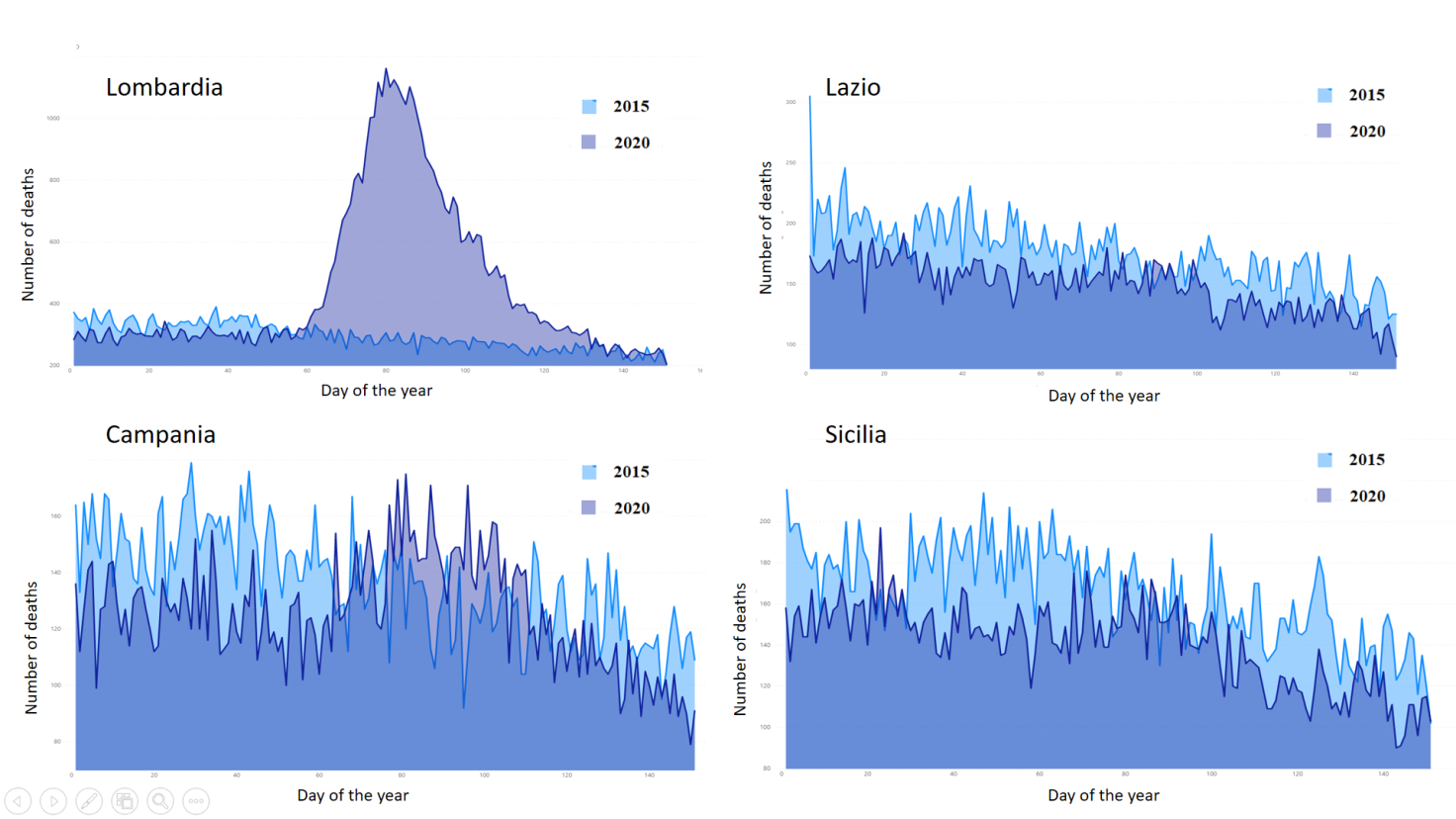
Data come from Istat, which provided information on mortality for 7,357 Italian municipalities (out of 7,904, 93.1%). [Source of data: Istat. Dati di mortalità: cosa produce l’ Istat. Decessi anni 2015-2020]
Co-morbidities in COVID-19 deceased patients
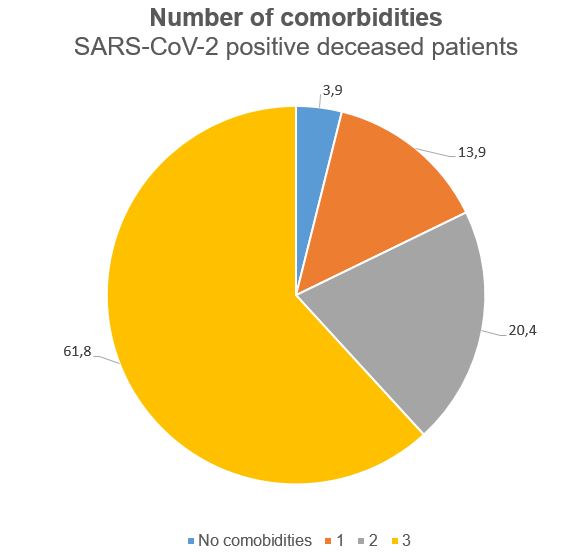
Older people with underlying conditions such as hypertension, heart and lung problems, diabetes or cancer are at greater risk of developing a severe form of the disease2. In Italy, the median age of affected individuals is 61 years while the median age of deceased patients is 81 years (85 years for women and 79 years for men). The median number of concomitant diseases in deceased patients is 3 (Figure 4); of these, the most frequent are arterial hypertension (66.2%), type 2 diabetes mellitus (29.8%), ischemic heart disease (27.7%) and arterial fibrillation (23%). The most common symptoms in deceased patients are fever, dyspnoea and cough whereas diarrhea and hemoptysis are less frequent12.
Figure 4 Distribution of number of comorbidities in SARS-CoV-2 positive deceased patients in Italy
The role of gender in COVID-19
Gender seems to play a very important role in this pandemic, in particular in regards to lethality, i.e. the percentage of confirmed deaths out of the total COVID-19 confirmed cases. According to bollettino della sorveglianza integrata COVID-19, updated on 14 July 2020 and published by Istituto Superiore di Sanità, in Italy male lethality from COVID-19 is higher than female lethality in all age groups, with the exception of the age group 0-9 years, with an overall male lethality of 17.6% and female lethality of 10.9%13.
Similar results were reported by many European and non-European countries that published COVID-19 sex-disaggregated data (with the exception of the Maldives, Nepal, Slovenia, Finland, Costa Rica and Israel)14 and were confirmed even after adjustment for age and several concomitant diseases (Hazard Ratio males vs females: 1.59 [95% CI: 1.53, 1.65; Figura 5])15.
Figure 5 Case-fatality rate in men over COVID-19 case-fatality rate in women
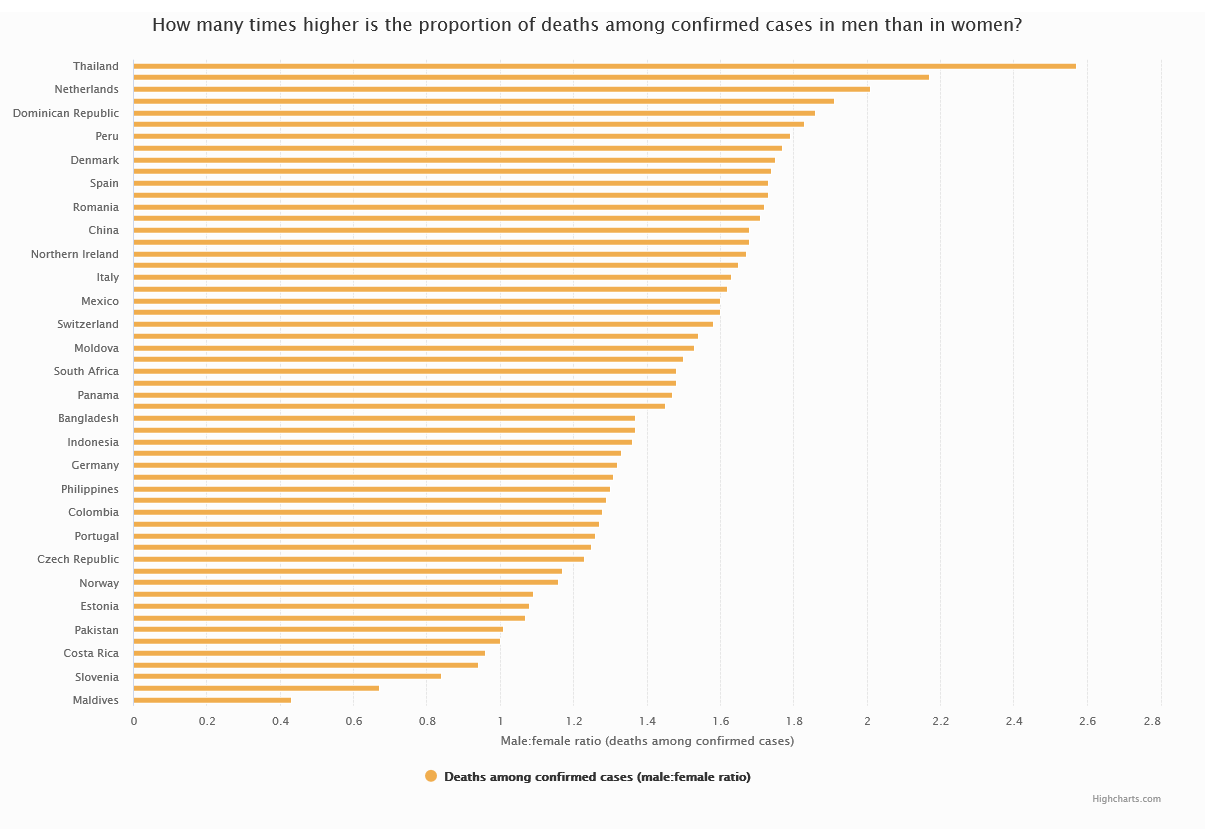
The chart shows the ratio of COVID-19 case-fatality rate in men over COVID-19 case-fatality rate in women for all the countries that had gender-disaggregated data available, as of 24 July 2020. [Source: COVID-19 sex-disaggregated data tracker, Global Health 5050.]
On the other hand, it is not yet known whether there is a possible role of gender in the spread of the Sars-CoV-2 virus. In Italy, 54.1% of COVID-19 confirmed cases are women, similarly to most of the other European countries, whereas in countries from Africa, Asia and South America there is a higher prevalence of males among the reported cases14. These data are probably influenced by the number of diagnostic tests carried out in the two groups of individuals and by the demographic characteristics of the population.
Another relevant question regards the number of COVID-19 cases among health workers. In Italy, COVID-19 affected health workers are 12.2% of total cases, have a median age of 48 years and 72.2% of them are women13. This result is similar to the one reported by other countries such as Spain (77%), the United States (73%), Germany (73%) and the Dominican Republic (64%)16 and could be explained by the higher prevalence of women working on the healthcare system. Despite this, these results also underline the importance of protective devices’ supplementation to these professional figures17.
COVID-19 and weather
Most respiratory viruses, including influenza, show a seasonal tendency to infection, with peaks of incidence during the winter season and a space-time transmission strongly associated with weather factors, such as atmospheric temperature and humidity18,19.
In 2010, three types of coronavirus – HCoV-HKU1, HCoV-NL63 and HCoV-OC43 – were identified to have significant winter seasonality, with the spread of the infection occurring mainly between December and April, very similarly to the influenza model20. The SARS-CoV epidemic, which spread in China in 2013, also peaked during the spring season, but its rapid containment did not allow to investigate the effect of seasonality on the spread. Conversely, the peak of MERS-CoV infections occurred during the summer season, mainly in a country characterized by hot weather such as Saudi Arabia21,22. In that case, high temperature and low humidity were associated with a higher incidence of cases23.
Given the variability of the evidence on the susceptibility of coronaviruses to climatic factors and given the limited data available on COVID-19, it is therefore complex to understand and make predictions on the evolution of the pandemic with the alternation of the next seasonal cycles.
Some laboratory studies, conducted under controlled conditions, detected a sensitivity of the Sars-CoV-2 virus to high temperatures. In particular, it was observed a reduction of 0.7 log-units in the virus transport medium (final concentration ~6.8 log-unit of 50% tissue culture infectious dose, TCID50, per mL) at 4 °C after 14 days of incubation, a reduction of over 3 log-units after 7 days of incubation at 22 °C and no detection of the virus after 14 days. At 37 °C, a reduction of over 3 log-units was observed after one day and no detection of the virus was found in the following days. Considering very high temperatures, the virus was no longer detected after 30 minutes at 56 ° C or after 5 minutes at 70 °C24.
Many epidemiological studies also investigated the role of temperature and other climatic factors on the spread of the virus, leading to conflicting results. Early studies based on data from China showed an inverse relationship between temperature, atmospheric humidity and the virus spread. A study conducted considering the cases from 30 regions of China between December 2019 and February 2020 observed that an increase of 1 °C in the average daily temperature was associated to a reduction from 36% to 57% in the daily-confirmed cases, when relative humidity was in a range between 67% and 87%. On the other hand, an increase of 1% of relative humidity was associated to a reduction from 11% to 22% of daily cases, when the average daily temperature was in the range between 5 °C and 8.2 °C. Despite this, these associations were not reproducible for all mainland China25. Similar results were also found for effective reproductive number, Rt, with an increase in temperature and relative humidity significantly associated with a reduction in the transmission rate, both before and after the implementation of lockdown measures26.
Starting from February 2020, the virus began to spread outside of China and reached several regions all over the world.
Immediately after China, Italy was the country most affected by the pandemic. An effect of climatic factors on the spread of the virus was suggested for Italy as well, as the northern regions, such as Lombardy, Veneto and Emilia Romagna, were hit by the virus much harder than the central and southern regions. A study conducted by Prof. Scafetta from Federico II University of Naples found that the winter climate that characterized the city of Wuhan in 2020 was very similar to that observed between February and March 2020 in Milan, Bergamo and Brescia, which are the Italian provinces most affected by COVID-1927. Several studies attempted to identify specific ranges of temperature and humidity conducive to the spread of the virus. It was observed that, between January 2020 and March 2020, Covid-19 growth rates peaked in the temperate regions of the Northern Hemisphere, and were lower in warmer and wetter or colder and drier regions28. In particular, the virus mainly spread within the 30–50° N corridor, in regions with average temperatures between 5–11 °C, combined with low specific humidity (3–6 g/kg) and low absolute humidity (4–7 g/m3)29.
A study conducted on the number of cases and deaths recorded in 166 countries around the world (excluding China) until March 27, 2020 found that temperature and relative humidity were inversely associated with both the number of new daily cases and the number of new daily deaths. The analysis also took into account possible confounding factors such as wind speed, median age of the population, the Global Health Security Index, the Human Development Index and population density. In particular, the authors observed that 1 °C increase was associated with a 3.1% (95% CI: 1.5%, 4.6%) reduction of new daily cases and a 1.2% (95% CI: 0.44%, 2.0%) reduction of new daily deaths, whereas 1% increase of relative humidity was associated with a 0.9% (95% CI: 0.5%, 1.2%) reduction of new daily cases and a 0.5% (95% CI : 0.34%, 0.67%) reduction of new daily deaths30. Similar results were reported by another study conducted on the number of confirmed cases up to 12 March 2020 in 310 regions of 116 countries in the world. They found that the incidence of COVID-19 was inversely associated not only with temperature and relative humidity, but also with wind speed and the UV index in the two weeks prior the incidence, i.e. before the incubation period of the virus31.
However, contrary to this evidence, recent works did not find any significant association between weather and the spread of the virus or reported a weak or non-geographically reproducible association. According to a not yet peer-reviewed study conducted on worldwide COVID-19 confirmed cases reported between December 31, 2019 and April 15, 2020, there is no evidence of an association between temperature and the transmission of the virus. The study, which also included the confirmed cases of some of the countries that recently faced the pandemic, such as Brazil, suggests that the significant associations found in previous studies may be biased by the fact that, initially, the virus spread mainly in the temperate areas of the northern hemisphere, which are also those characterized by greater human mobility32.
A study by University of Toronto investigated the effect of climatic factors on the number of confirmed cases between March 20, 2020 and March 27, 2020 in 144 countries. In multivariate analysis, which took into account several socio-demographic confounders and information on public health interventions, they found that temperature, latitude and absolute humidity were not associated with epidemic growth33.
A recent work carried out in collaboration between several research groups and not yet peer-reviewed evaluated how temperature, humidity, exposure to ultraviolet rays and other climatic factors have influenced the effective reproduction number Rt in almost 4000 locations around the world. Although the high temperature and increased exposure to ultraviolet rays were found to have a modest effect on the spread of Sars-CoV-2, the study concludes that this is not sufficient to control the evolution of the pandemic34.
Similar conclusions were reached by the National Academies of Sciences, Engineering and Medicine, which called for caution in the interpretation of these results35.
All the studies that attempted to investigate a possible seasonality of Sars-CoV-2 virus have, indeed, several limitations and are strongly influenced both by the short time window to which the COVID-19 data refer and by the fact that the first phase of the epidemic, which started from China, was limited to northern regions of the hemisphere. The quality of the data and the different classification and communication criteria of the COVID-19 cases adopted by countries may also have influenced the results.
There are also several confounding factors related to the geography (and, therefore, to the temperature and the weather) that could better explain the transmission of the virus and which are, at the same time, very complex to measure and control in epidemiological studies. Some examples are the type of healthcare system, the type of adopted containment measures, the possibility to access treatment, behavioral habits, mobility within and outside the country and travel restrictions. Regarding this, some studies have observed that air transport has contributed to the spread of the virus36,37 also in case of travellers’ screening38, whereas travel restriction practices applied by many countries have slowed down the spread of the virus39,40.
Consequently, limiting the investigation on changes in climatic factors may not provide reliable results and only relying on the arrival of the hot season without planning and implementing targeted health policies may not be sufficient to contain the virus transmission41,42.
Although there is evidence that suggest a potential reduction in cases in the hottest and wetter regions, we should be aware that these results are not enough to establish a causal link. Currently, we are witnessing a rapid spread of the virus in areas of the southern hemisphere and in hot countries that are experiencing the summer season, such as India, Iran and several states of the United States, and the data from these countries will be essential to better understand the influence of climatic factors on the transmission of Sars-CoV-2.
COVID-19 and pollution
The association between pollution and COVID-19 is a topic highly debated in the scientific community, given that some of the areas most affected by the pandemic, such as the Chinese region of Hubei, the Po Valley and the city of New York, are also regions characterized by high levels of air pollution. This led experts to wonder whether there is a cause-effect link between exposure to pollution and COVID-19, both in the spread of the virus and in the prognosis of diseases related to SARS-CoV-2 virus infection.
A Position Paper presented by the Italian Society of Environmental Medicine (SIMA) in mid-March hypothesized that PM10 atmospheric particulate matter may have a role as carrier of the virus both directly and through a boost action, i.e. impulse to diffusion. These conclusions were drawn after observing a strong correlation between the number of COVID-19 cases recorded in the Italian provinces on 3 March 2020 and the number of exceedances of the PM10 concentrations limit allowed by the law in the period between February 10, 2020 and February 29, 202043.
A second study by SISMA also identified traces of the virus RNA in several PM10 samples collected in industrial sites in the province of Bergamo between 21 February and 13 March, leading the authors to hypothesize a possible aerial transmission of the virus through particulate matter44. These positions, which had a strong media impact, led several research institutions45,46 and researchers47 to express doubts about the methodology used and to call for caution in interpreting these results.
In fact, although the presence of biological particles such as bacteria, fungi and viruses in the atmospheric particulate has been known for some time48, there is no evidence that Coronaviruses are able to maintain an infectious viral load for a prolonged time in an outdoor environment49. For this reason, based on current evidence, experts tend not to believe that atmospheric particulate matter can be a “carrier” of the virus.
At the same time, it is now known that exposure to high concentrations of atmospheric particulate matter and NO2 is correlated to an higher prevalence of respiratory and cardiovascular diseases, with a consequent greater susceptibility to respiratory infections and greater severity in the manifestation of symptoms48.
According to the World Health Organization, air pollution causes about 4.2 million premature deaths each year. In 2016, 58% of these deaths were due to ischemic heart disease and stroke, 18% to chronic obstructive pulmonary disease and acute lower respiratory tract infections, and 6% to lung cancer50.
Based on this evidence, one of the questions that experts are trying to find an answer to is whether exposure to air pollution could lead to a more severe form of Sars-CoV-2 infection and, consequently, to a greater risk of death.
The hypothesis is that chronic exposure to atmospheric particulates can activate an inflammation process and lead to the onset of more severe symptoms. In fact, several studies have shown that both PM2.5 and PM10 cause systemic inflammation in humans with an overexpression of several cytokines, including IL8, IL17, IL6, TNFα. These alterations, in addition to contributing to the onset of cardio-respiratory diseases, lead to a weakening of the immune system and, consequently, to an acute respiratory distress syndrome and, in the worst case scenario, to death51.
Some epidemiological studies have also investigated the possible association between air pollution and COVID-19 mortality.
Particular attention by media was paid to a not yet peer-reviewed study by Harvard University, which investigated the association between long-term exposure to PM2.5 and COVID-19 mortality. The analysis was carried out considering about 3000 counties of the United States (equal to 98% of the total coverage of the territory) and published in two versions. In the first version, a multivariate analysis was carried out considering the number of COVID-19 confirmed deaths reported by US counties up to April 4, 2020, and various confounders related to socio-demographic factors (such as population density, percentage of over 65, percentage of poor, ethnicity, income and others) and climatic conditions (average winter temperature, average summer temperature, average winter relative humidity, average summer relative humidity) were taken into account. The results of this first analysis showed that the 1 µg/m3 increase in PM2.5 was associated with 15% (95% CI: 5%, 25%) increase in risk of COVID-19 mortality.
In the second version, the analysis was updated considering the confirmed COVID-19 deaths recorded up to 22 April 2020 and further confounders were added to the analysis, regarding the time of virus spread and implementation of social distancing policies in each county, as well as further details on the age distribution of the population. Adding this new information in the analysis significantly reduced the strength of the association between atmospheric particulate matter and COVID-19 mortality, leading to a halving of the mortality rate compared to the initial analysis (Mortality Rate Ratio = 8%, 95% CI: 2%, 15%)52.
These results, which provided many inputs to the scientific debate, have, however, important limitations, typical of ecological studies. In fact, studying the long-term effects of air pollution on health requires the use of aggregated data, not only as regards the counting of confirmed deaths but also as regards environmental measurements. Assigning a PM2.5 summary measurement to a given geographical area implies not considering spatial autocorrelation and that means excluding all information deriving from geographical variability that could, in some way, modulate the effect of the confounding factors on COVID-19 mortality. Furthermore, as observed in the comparison between the results of the two versions of the analysis, it is important to take into account the mechanisms of the virus spread and information on containment policies, in order to obtain an unbiased assessment of the role played by air pollution within this pandemic and to avoid getting spurious associations. To do this, it is necessary to carry out properly planned studies, which prospectively evaluate the role played by exposure to air pollution on the prognosis of COVID-19, both independently and by interacting with other possible confounding factors.
Conclusions
Some studies suggest that Sars-CoV-2 virus spreads less in wet and warm areas and more easily in cold and dry regions, suggesting a seasonal effect on transmission. These studies, however, have several sources of bias and limitations, both from a methodological point of view and in the data quality. For these reasons, further investigations are needed.
In addition, experts urge caution in interpreting these results, as no country has yet been exposed to the virus for an entire year of seasonal variations; therefore, all assumptions are based on evidence coming from other infectious diseases or other strains of coronavirus, and on limited COVID-19 data.
Exposure to high levels of air pollution also seems to be associated with a worse prognosis of the disease but studies that takes into account the several sources of bias are needed to better investigate this association.
References
- WHO Coronavirus Disease (COVID-19) Dashboard | WHO Coronavirus Disease (COVID-19) Dashboard.
- WHO. Q&A on coronaviruses (COVID-19). Who. 2020. p. 1–15.
- Coronavirus Symptoms: Frequently Asked Questions | Johns Hopkins Medicine.
- Ghani AC, Donnelly CA, Cox DR, Griffin JT, Fraser C, Lam TH, et al. Methods for estimating the case fatality ratio for a novel, emerging infectious disease. Am J Epidemiol . 2005;162(5):479–86.
- Wilder-Smith A, Freedman DO. Confronting the New Challenge in Travel Medicine: SARS . Vol. 10, Journal of Travel Medicine. 2003.
- Roser M, Ritchie H, Ortiz-Ospina E, Hansell J. Mortality Risk of COVID-19 – Statistics and Research – Our World in Data. Mortality Risk of COVID-19. 2020. p. 1. Ultimo accesso: 25 luglio 2020.
- Oke J, Heneghan C. Global Covid-19 Case Fatality Rates. OXFORD CEBM Research. 2020. p. 1–12. Ultimo accesso: 25 luglio 2020.
- Sudharsanan N, Didzun O, Bärnighausen T, Geldsetzer P. The Contribution of the Age Distribution of Cases to COVID-19 Case Fatality Across Countries: A 9-Country Demographic Study. Ann Intern Med. 2020 Jul 22;M20-2973.
- Istituto superiore di sanità (ISS). La mortalità – dati – registri – sorveglianza. 2020.
- Scortichini M, Santos RS dos, Donato FD, Sario M De, Michelozzi P, Davoli M, et al. Excess mortality during the COVID-19 outbreak in Italy: a two-stage interrupted time series analysis. medRxiv. 2020 Jul 24;2020.07.22.20159632.
- Michelozzi P, De’ Donato F, Scortichini M, De Sario M, Asta F, Agabiti N, et al. On the increase in mortality in italy in 2015: Analysis of seasonal mortality in the 32 municipalities included in the surveillance system of daily mortality. Epidemiol Prev. 2016;40(1):22–8.
- Istituto Superiore di Sanità (ISS). Report sulle caratteristiche dei pazienti deceduti positivi all’infezione da SARS-CoV-2 in Italia. Aggiornamento del 22 luglio 2020.
- Riccardo F, Andrianou X, Bella A, Del Manso M, Mateo Urdiales A, Fabiani M, et al. Bollettino della sorveglianza integrata COVID-19. Aggiornamento 25 agosto 2020. Istituto Superiore di Sanità (ISS).
- Global Health 5050. COVID-19 sex-disaggregated data tracker – Global Health 50/50 . 2020. Ultimo accesso: 25 luglio 2020.
- Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. OpenSAFELY: factors associated with COVID-19 death in 17 million patients. Nature . 2020 Jul 8;1–11.
- Global Health 5050. Healthcare workers – Global Health 50/50. Ultimo accesso: 25 luglio 2020.
- Istituto Superiore di Sanità. Gender differences in COVID-19: the importance of sex-disaggregated data. Epicentro. 2020
- Shaman J, Kohn M. Absolute humidity modulates influenza survival, transmission, and seasonality. Proc Natl Acad Sci U S A. 2009 Mar 3;106(9):3243–8.
- Price RHM, Graham C, Ramalingam S. Association between viral seasonality and meteorological factors. Sci Rep. 2019 Dec 1;9(1):1–11.
- Gaunt ER, Hardie A, Claas ECJ, Simmonds P, Templeton KE. Epidemiology and Clinical Presentations of the Four Human Coronaviruses 229E, HKU1, NL63, and OC43 Detected over 3 Years Using a Novel Multiplex Real-Time PCR Method. J Clin Microbiol. 2010;48(8):2940–7.
- Nassar MS, Bakhrebah MA, Meo SA, Alsuabeyl MS, Zaher WA. Global seasonal occurrence of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection. Vol. 22, European Review for Medical and Pharmacological Sciences. 2018. p. 3913–8.
- Al-Ahmadi K, Alahmadi S, Al-Zahrani A. Spatiotemporal clustering of middle east respiratory syndrome coronavirus (MERS-CoV) incidence in Saudi Arabia, 2012–2019. Int J Environ Res Public Health. 2019 Jul 2;16(14).
- Altamimi A, Ahmed AE. Climate factors and incidence of Middle East respiratory syndrome coronavirus. J Infect Public Health. 2020 May 1;13(5):704–8.
- Chin AWH, Chu JTS, Perera MRA, Hui KPY, Yen H-L, Chan MCW, et al. Stability of SARS-CoV-2 in different environmental conditions. The Lancet Microbe. 2020 May 1;1(1):e10.
- Qi H, Xiao S, Shi R, Ward MP, Chen Y, Tu W, et al. COVID-19 transmission in Mainland China is associated with temperature and humidity: A time-series analysis. Sci Total Environ. 2020 Aug 1;728:138778.
- Wang J, Tang K, Feng K, Li X, Lv W, Chen K, et al. High Temperature and High Humidity Reduce the Transmission of COVID-19. SSRN Electron J. 2020 Mar 9.
- Scafetta N. Distribution of the SARS-CoV-2 pandemic and its monthly forecast based on seasonal climate patterns. Int J Environ Res Public Health. 2020 May 17;17(10):3493.
- Ficetola GF, Rubolini D. Climate affects global patterns of COVID-19 early outbreak dynamics. medRxiv. 2020 Apr 20;2020.03.23.20040501.
- Sajadi MM, Habibzadeh P, Vintzileos A, Shokouhi S, Miralles-Wilhelm F, Amoroso A. Temperature, Humidity, and Latitude Analysis to Estimate Potential Spread and Seasonality of Coronavirus Disease 2019 (COVID-19). JAMA Netw open. 2020 Jun 1;3(6):e2011834.
- Wu Y, Jing W, Liu J, Ma Q, Yuan J, Wang Y, et al. Effects of temperature and humidity on the daily new cases and new deaths of COVID-19 in 166 countries. Sci Total Environ. 2020 Aug 10;729:139051.
- Islam N, Shabnam S, Erzurumluoglu AM. Temperature, humidity, and wind speed are associated with lower Covid-19 incidence. medRxiv. 2020 Mar 31;2020.03.27.20045658.
- Jamil T, Alam IS, Gojobori T, Duarte C. No Evidence for Temperature-Dependence of the COVID-19 Epidemic. medRxiv. Cold Spring Harbor Laboratory Press; 2020 Apr.
- Jüni P, Rothenbühler M, Bobos P, Thorpe KE, Da Costa BR, Fisman DN, et al. Impact of climate and public health interventions on the COVID-19 pandemic: A prospective cohort study. CMAJ. 2020 May 25;192(21):E566–73.
- Xu R, Rahmandad H, Gupta M, DiGennaro C, Ghaffarzadegan N, Amini H, et al. The Modest Impact of Weather and Air Pollution on COVID-19 Transmission. medRxiv. 2020.
- Rapid Expert Consultation on SARS-CoV-2 Survival in Relation to Temperature and Humidity and Potential for Seasonality for the COVID-19 Pandemic (April 7, 2020). National Academies Press; 2020.
- Gilbert M, Pullano G, Pinotti F, Valdano E, Poletto C, Boëlle PY, et al. Preparedness and vulnerability of African countries against importations of COVID-19: a modelling study. Lancet. 2020 Mar 14;395(10227):871–7.
- Peeri NC, Shrestha N, Rahman MS, Zaki R, Tan Z, Bibi S, et al. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: what lessons have we learned? IEA Int Epidemiol Assoc Int J Epidemiol. 2020:1–10.
- Gostic KM, Gomez ACR, Mummah RO, Kucharski AJ, Lloyd-Smith JO. Estimated effectiveness of symptom and risk screening to prevent the spread of COVID-19. Elife. 2020;9.
- Chinazzi M, Davis JT, Ajelli M, Gioannini C, Litvinova M, Merler S, et al. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science (80- ). 2020 Apr 24;368(6489):395–400.
- Lee VJ, Chiew CJ, Khong WX. Interrupting transmission of COVID-19: lessons from containment efforts in Singapore. J Travel Med. 2020;2020:1–5.
- Luo W, Majumder MS, Liu D, Poirier C, Mandl KD, Lipsitch M, et al. The role of absolute humidity on transmission rates of the COVID-19 outbreak. medRxiv. 2020 Feb 17;7.
- Poirier C, Luo W, Majumder M, Liu D, Mandl K, Mooring T, et al. The Role of Environmental Factors on Transmission Rates of the COVID-19 Outbreak: An Initial Assessment in Two Spatial Scales. SSRN Electron J. 2020 Mar 13.
- Setti L. Evaluation of the potential relationship between Particulate Matter (PM) pollution and COVID-19 infection spread in Italy. Società Italiana di Medicina Ambientale (SIMA).
- Setti L, Passarini F, De Gennaro G, Barbieri P, Perrone MG, Borelli M, et al. SARS-Cov-2RNA found on particulate matter of Bergamo in Northern Italy: First evidence. Environ Res. 2020 Sep 1;188:109754.
- Società Italiana di Aerosol. Information on the relationship between air pollution and the spread of COVID-19.
- Carla Ancona PALB et. altri (2020). Inquinamento atmosferico e epidemia COVID-19: la posizione della Rete Italiana Ambiente e Salute – E&P Repository.
- Contini D, Costabile F. Does air pollution influence COVID-19 outbreaks?. Vol. 11, Atmosphere. MDPI AG; 2020. p. 377.
- Baldini M, Bartolacci S, Bortone G, et. al (2020). Valutazione del possibile rapporto tra l’inquinamento atmosferico e la diffusione del SARS-CoV-2. E&P Repository.
- Rete Italiana Ambiente e Salute. Inquinamento atmosferico e COVID-19: la posizione della Rete Italiana Ambiente e Salute | Scienza in rete.
- WHO. Ambient (outdoor) air pollution. 2018.
- Conticini E, Frediani B, Caro D. Can atmospheric pollution be considered a co-factor in extremely high level of SARS-CoV-2 lethality in Northern Italy? Vol. 261, Environmental Pollution. Elsevier Ltd; 2020. p. 114465.
- Wu X, Nethery RC, Sabath BM, Braun D, Dominici F. Exposure to air pollution and COVID-19 mortality in the United States. medRxiv. 2020 Apr 27;2020.04.05.20054502.



 Scarica il PDF dell'articolo
Scarica il PDF dell'articolo
Commenti